Several years ago my Veterinarian prescribed glucosamine chondroitin to help relieve Sena’s stiff joints. At $90 per bottle, I found comparable over-the-counter pet supplements for just $10 per bottle. My Vet told me not to use those because pet supplements are not regulated and may not have any active ingredient at all. She recommended the prescription version because it's been voluntarily tested and the level of glucosamine chondroitin stated on the label confirmed. I assumed the quality control issue was isolated to pet supplements. Certainly not human supplements, right?
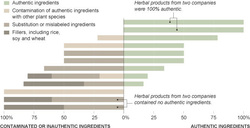
In a research article published last month by BMC Medicine (see resources) the authors used DNA barcoding, a kind of DNA fingerprinting, to test 44 herbal products from 12 different companies for authenticity. They were able to authenticate 48%, not even half of the products tested. More than half (59%) of the products tested contained species of plants that were not listed on the labels. About 1/3 of the authenticated herbal products also contained contaminants and/or fillers that were not listed on the label.
Only two of the 12 herbal companies provided authentic products with no substitution, contaminants, or fillers. Most (75%) of the herbal companies did have some authentic products. The authors were unable to authenticate any products for 25% of the companies.
Contamination and substitution in herbal products pose significant health risks to consumers. The authors found contamination with plants that have known toxicity, side effects, or that interact negatively with other herbs, supplements, or medications. Several of the herbal products were contaminated with feverfew, an invasive weed native to Eurasia that has negative side effects and should not be consumed by pregnant women. A Ginkgo product was contaminated with black walnut, which is dangerous for consumers with nut allergies, and the jugline in walnut leaves is toxic.
The authors found substitution in 30 out of the 44 products tested (68%). In one case, a St. John’s wort product turned out to be substituted with senna, an herbal laxative that is not intended for long-term use and can cause adverse effects. In another case the authors found substitution of black cohosh for Asian Actaea.
Unlabeled plant fillers are commonly used in supplements, including rice, soybean, and various grasses such as wheat and alfalfa.
Although supplements are not regulated, I just assumed that supplements for humans were held to somewhat higher standards. But apparently it's all gone to the dogs...
Resources
Only two of the 12 herbal companies provided authentic products with no substitution, contaminants, or fillers. Most (75%) of the herbal companies did have some authentic products. The authors were unable to authenticate any products for 25% of the companies.
Contamination and substitution in herbal products pose significant health risks to consumers. The authors found contamination with plants that have known toxicity, side effects, or that interact negatively with other herbs, supplements, or medications. Several of the herbal products were contaminated with feverfew, an invasive weed native to Eurasia that has negative side effects and should not be consumed by pregnant women. A Ginkgo product was contaminated with black walnut, which is dangerous for consumers with nut allergies, and the jugline in walnut leaves is toxic.
The authors found substitution in 30 out of the 44 products tested (68%). In one case, a St. John’s wort product turned out to be substituted with senna, an herbal laxative that is not intended for long-term use and can cause adverse effects. In another case the authors found substitution of black cohosh for Asian Actaea.
Unlabeled plant fillers are commonly used in supplements, including rice, soybean, and various grasses such as wheat and alfalfa.
Although supplements are not regulated, I just assumed that supplements for humans were held to somewhat higher standards. But apparently it's all gone to the dogs...
Resources



 RSS Feed
RSS Feed