Being an analytical chemist and specializing in chemical measurements, I decided to find out what assays are used, how they work, and what actually gets measured. There are several different assays, with each one being a little different, and none of which measure total antioxidant activity. For today, I’m only going to tell you about one of those assays, the Ferric Reducing Antioxidant Power (FRAP) assay.
The broad definition of an antioxidant is any substance that delays or stops oxidative damage to a target molecule (free radical). Antioxidants accomplish this by neutralizing free radicals, which they do by transferring an electron to the free radical (also known as “reducing” the free radical).
The FRAP assay is a fast and simple method for measuring the antioxidant power of foods and beverages. This assay uses antioxidant standards, solutions that have known amounts (concentrations) of antioxidants. The antioxidant standards are mixed with an iron (Fe) complex (FeIII-TPTZ) that serves as the target molecule (free radical). The FeIII-TPTZ in solution is a colorless liquid and acts as a free radical by accepting an electron from the antioxidant that it’s mixed with. When the FeIII-TPTZ complex takes an electron from the antioxidant, it gets converted to a different form (FeII) that is blue-colored. The change in color of the liquid from colorless to blue can be accurately measured (as a change in absorbance at 593 nm) after a reaction time of four minutes, which allows all antioxidants in the sample to react with the FeIII-TPTZ complex.
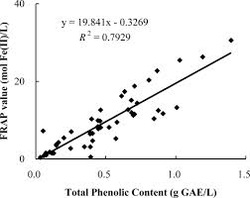
Here’s how: the experiment is repeated, only this time the antioxidants present in food extracts are mixed with the FeIII-TPTZ complex instead of the antioxidant standards. The FeIII-TPTZ complex changes to the FeII form when the antioxidants in the food extracts give an electron to the FeIII-TPTZ complex (in other words, the antioxidants neutralize the “free radical”) and the solution changes from colorless to blue. Just like before with the standards, the color change is measured as a change in absorbance at 593 nm. Now the calibration curve is used to determine the antioxidant concentration by plotting concentration of the FeII form of the food extract and using that to find the corresponding antioxidant concentration. All the plotting and calculations are done by computer, so they’re quite accurate.
At least now you have some idea about how claims can be made about the antioxidant power of foods. Stay tuned for future posts on some of the other methods.



 RSS Feed
RSS Feed